Akeso's Clinical Trial Failure Sends Stock Prices Tumbling
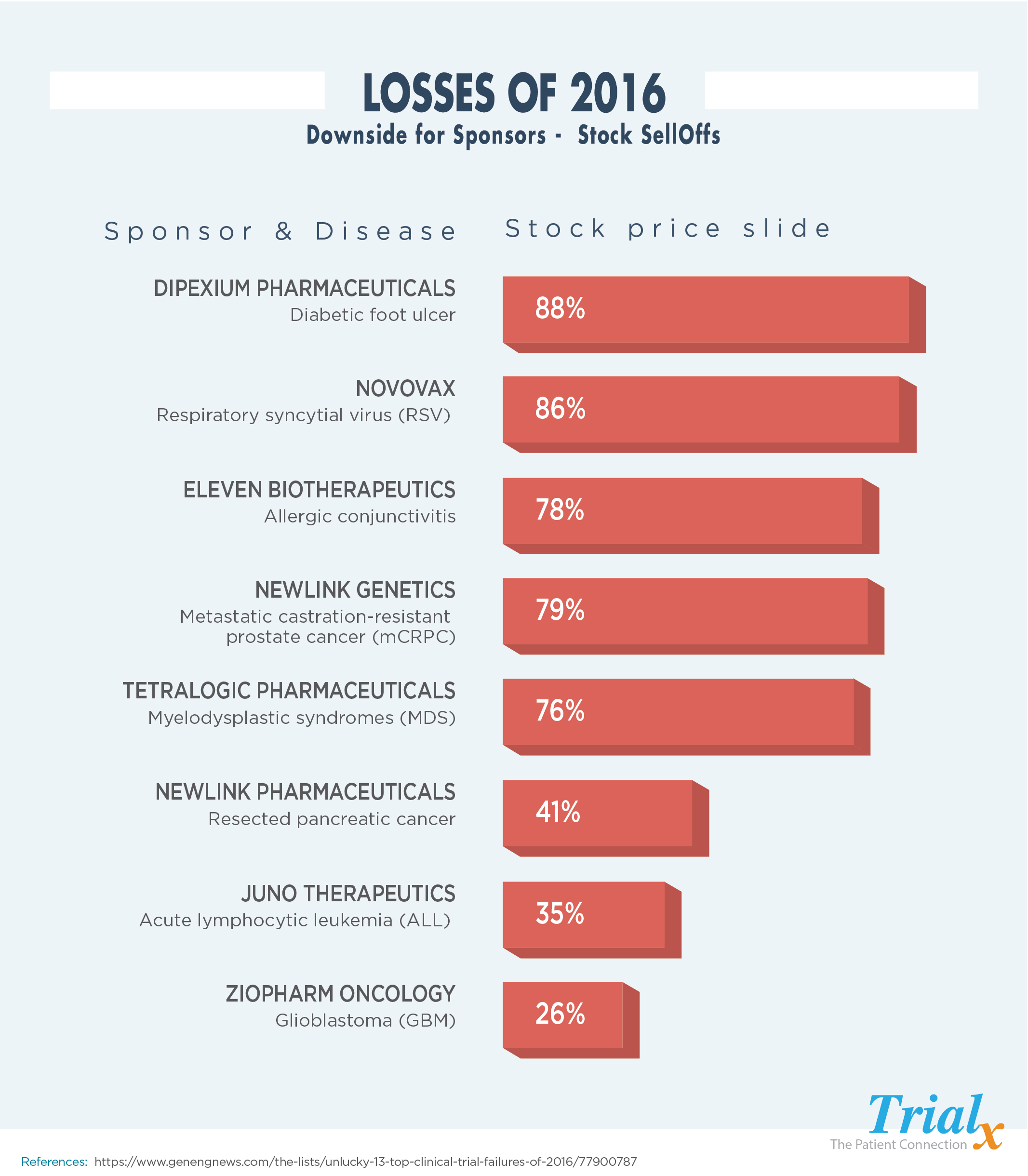
Table of Contents
The Failed Clinical Trial: Details and Implications
The failed clinical trial was a Phase III study evaluating [Drug Name], a novel [Drug Class] designed to treat [Specific Cancer Type]. The trial's failure stemmed from [Reason for Failure – e.g., a lack of statistically significant improvement in overall survival compared to the control group, p-value > 0.05, or unacceptable safety concerns]. This outcome represents a significant setback for Akeso and its investors.
- Key Findings: The trial failed to meet its primary endpoint of [Primary Endpoint, e.g., improved progression-free survival]. Secondary endpoints also showed [Results of Secondary Endpoints].
- Impact on Timeline: The failure necessitates a complete reassessment of the drug's development timeline, likely delaying or halting its potential market entry. Further research and potentially new clinical trials will be required.
- Regulatory Repercussions: The failure could lead to regulatory scrutiny and potential challenges in obtaining approval for [Drug Name] in the future. This uncertainty adds further pressure on Akeso.
Market Reaction: Stock Price Crash and Investor Sentiment
The announcement of Akeso's clinical trial failure triggered an immediate and severe market reaction. The company's stock price plummeted by [Percentage]% in a single day, wiping out [Dollar Amount] in market capitalization. Trading volume surged as investors rushed to sell their shares.
- Stock Price Before/After: Before the announcement, Akeso's stock was trading at [Price]; after the news, it fell to [Price].
- Trading Volume Spike: Trading volume increased by [Percentage]%, reflecting the heightened investor activity.
- Market Capitalization Change: The company's market capitalization decreased by [Dollar Amount].
- Analyst Comments: Financial analysts expressed concerns about Akeso's future prospects, with some downgrading their investment ratings. [Quote from a reputable financial analyst].
Akeso's Response and Future Outlook
In response to the clinical trial failure, Akeso released an official statement [Link to Press Release] acknowledging the disappointing results and outlining its next steps. The company stated its commitment to [Future Plans, e.g., analyzing the data to understand the reasons for failure, exploring alternative strategies, and evaluating the viability of further development of the drug].
- Official Press Release: Akeso's statement emphasized the importance of patient safety and its dedication to ongoing research.
- Future Clinical Trials: The company may initiate further clinical trials to explore alternative formulations or treatment strategies.
- Company Restructuring: Akeso might need to restructure its operations to address the financial implications of the failure.
- Potential Partnerships: Seeking strategic partnerships or collaborations could be a crucial step for Akeso to navigate this challenging period.
Wider Implications for the Biopharmaceutical Industry
Akeso's clinical trial failure underscores the inherent risks associated with biopharmaceutical drug development. It serves as a stark reminder of the high failure rate in bringing new drugs to market and the significant financial implications of setbacks.
- Impact on Investor Confidence: The event could lead to a decline in investor confidence in other biopharmaceutical companies with similar drug candidates.
- Regulatory Implications: Regulatory agencies may review their processes in light of this failure.
- Lessons Learned: Other companies can learn valuable lessons from analyzing the reasons behind Akeso's failure to improve their own development processes.
- Changes in Investment Strategies: Investors might re-evaluate their investment strategies in the biopharmaceutical sector, potentially shifting towards companies with more diversified pipelines or less risky drug development profiles.
Conclusion: Understanding the Risks Associated with Akeso's Clinical Trial Failure
Akeso's clinical trial failure is a significant event with far-reaching consequences. The dramatic stock price drop, Akeso's response, and the wider implications for the industry highlight the inherent uncertainties and risks involved in drug development. Understanding the reasons behind this setback is crucial for Akeso, its investors, and the entire biopharmaceutical industry. To stay informed about Akeso's progress and the evolving landscape of biopharmaceutical investment, follow financial news closely, conduct thorough due diligence, and consider diversifying your investment portfolio. Monitoring Akeso's clinical trial results and other drug development setbacks will be crucial for making informed investment decisions in this volatile sector.

Featured Posts
-
 Why Is Porsche More Popular Internationally Than In Australia
Apr 29, 2025
Why Is Porsche More Popular Internationally Than In Australia
Apr 29, 2025 -
 Pete Rose Pardon Trumps Posthumous Gesture Explained
Apr 29, 2025
Pete Rose Pardon Trumps Posthumous Gesture Explained
Apr 29, 2025 -
 The Brain Drain Trumps Funding Cuts And The Global Race For American Scientists
Apr 29, 2025
The Brain Drain Trumps Funding Cuts And The Global Race For American Scientists
Apr 29, 2025 -
 Where To Invest Mapping The Countrys Top Business Destinations
Apr 29, 2025
Where To Invest Mapping The Countrys Top Business Destinations
Apr 29, 2025 -
 Obnova Konania V Pripade Unosu Studentky Sone Sudna Rozprava V Stredu
Apr 29, 2025
Obnova Konania V Pripade Unosu Studentky Sone Sudna Rozprava V Stredu
Apr 29, 2025
