Trump's FDA And Biotech: A Bullish Market Signal?
Table of Contents
Deregulation and its Impact on Biotech Innovation
The Trump administration's focus on deregulation significantly altered the landscape of drug development and approval. This had profound implications for biotech innovation, accelerating timelines and potentially boosting investment.
Accelerated Drug Approval Processes
Initiatives to streamline drug approvals under the Trump administration aimed to reduce the time it took for new treatments to reach patients.
- Examples of faster approvals: Several drugs received accelerated approval under the Trump administration, some under the controversial Breakthrough Therapy designation. This led to faster market entry for innovative treatments. Specific examples would require detailed analysis of FDA approval data during that period.
- Changes in review processes: The FDA implemented changes to its review processes, aiming for greater efficiency and collaboration with industry stakeholders. This included a focus on utilizing data from real-world evidence alongside traditional clinical trials.
- Impact on specific biotech companies: Companies focused on oncology and other high-need areas potentially benefited the most from faster approvals, leading to quicker revenue streams and increased valuations.
Reduced Regulatory Burden
Reducing bureaucratic hurdles was a central theme of the Trump administration's approach to regulation. This had a noticeable effect on both established biotech companies and smaller startups.
- Examples of reduced paperwork: Streamlining applications and reducing unnecessary paperwork freed up resources for biotech firms, allowing them to focus on research and development.
- Faster clinical trial approvals: Faster approvals for clinical trials meant less time spent on navigating regulatory processes and more time conducting crucial research.
- Impact on funding and investment: The perceived reduced regulatory risk made the biotech sector more attractive to investors, leading to an increase in venture capital funding and initial public offerings (IPOs).
Right-to-Try Initiatives
The expansion of Right-to-Try initiatives, allowing terminally ill patients access to experimental treatments, also impacted the biotech sector.
- Ethical considerations: The Right-to-Try movement sparked debate regarding ethical considerations, patient safety, and the balance between providing access and ensuring rigorous scientific evaluation.
- Patient access: Increased patient access to experimental treatments may have improved public perception of the biotech industry, indirectly influencing investment.
- Market response to Right-to-Try legislation: The market response to Right-to-Try legislation was mixed, with some seeing it as a positive signal of patient-centricity, while others expressed concerns about potential risks.
Market Response to Trump's FDA Policies
The impact of the Trump administration's FDA policies on the biotech market was multifaceted and requires a nuanced analysis.
Stock Market Performance of Biotech Companies
The stock market performance of biotech companies during the Trump administration needs to be analyzed in the context of overall market conditions.
- Charts showing stock market trends: A detailed analysis would require charting the performance of various biotech indices and individual company stocks during this period, comparing their performance with other market sectors.
- Correlation between FDA policies and stock prices: Establishing a direct correlation between specific FDA policies and stock price movements is challenging due to the multiple factors influencing market performance.
- Specific examples of companies that benefited: Identifying specific companies that demonstrably benefited from accelerated approvals or reduced regulatory burden would help illustrate the impact of these policies.
Increased Biotech Investment
The Trump administration's policies arguably contributed to an increase in investment in the biotech sector.
- Venture capital investments: Data on venture capital investments in biotech during the Trump years would show whether there was a noticeable increase compared to previous periods.
- IPOs: The number and success of biotech IPOs could provide another metric for assessing the market's response to the regulatory changes.
- Mergers and acquisitions (M&A) activity: Increased M&A activity could reflect a positive market outlook, driven by increased investment and confidence in the biotech sector.
International Implications
Trump's FDA policies also had international implications, affecting global collaborations and investment in the US biotech sector.
- Changes in global regulatory harmonization: The Trump administration's approach to international cooperation could have influenced the pace of global regulatory harmonization, potentially impacting cross-border collaborations.
- Impact on foreign investment in US biotech: Foreign investment in US biotech companies may have been affected by the perceived regulatory climate and political uncertainty.
- Cross-border collaborations: The level of cross-border collaborations between US and international biotech firms may have changed as a result of the administration's policies.
Counterarguments and Criticisms
While the Trump administration's policies had positive aspects, criticisms regarding safety and political influence warrant consideration.
Concerns Regarding Safety and Efficacy
Accelerated approvals, while potentially beneficial for patients needing faster access to treatments, raised concerns about patient safety and long-term efficacy.
- Examples of drug recalls or adverse events: Any instances of drug recalls or significant adverse events following accelerated approvals should be analyzed to assess potential risks.
- Concerns about long-term efficacy: The long-term efficacy of drugs approved under accelerated pathways needs careful monitoring.
- Counterarguments from regulatory experts: It's crucial to consider counterarguments from regulatory experts who may have concerns about the balance between speed and safety in drug approvals.
Political Polarization and its Influence
Political polarization played a role in shaping the regulatory environment and investor sentiment.
- Impact of political uncertainty: Political uncertainty surrounding FDA leadership and policy changes could have created instability in the biotech market.
- Changes in FDA leadership: Frequent changes in FDA leadership under the Trump administration might have added to regulatory uncertainty.
- Influence of partisan politics on regulatory decisions: Concerns about partisan politics influencing regulatory decisions could have negatively impacted investor confidence.
Conclusion
The impact of Trump's FDA and Biotech policies remains a complex issue. While initiatives aimed at accelerating drug approvals and reducing regulatory burdens potentially stimulated innovation and investment, concerns about safety and the influence of political polarization need careful consideration. The net effect on the biotech market is difficult to definitively quantify, requiring further in-depth analysis of specific companies, market trends, and long-term health outcomes. Further research into the long-term effects of these policies on the biotech industry is crucial for investors. Continue your research into the complex relationship between Trump's FDA and Biotech to make informed investment decisions. Consider the long-term impact of regulatory changes on your portfolio.

Featured Posts
-
 Is Your Florida Condo Difficult To Sell Understanding Current Market Conditions
Apr 23, 2025
Is Your Florida Condo Difficult To Sell Understanding Current Market Conditions
Apr 23, 2025 -
 Is Ai Revolutionizing Wildlife Conservation Examining The Benefits And Drawbacks
Apr 23, 2025
Is Ai Revolutionizing Wildlife Conservation Examining The Benefits And Drawbacks
Apr 23, 2025 -
 Trump Meets With Walmart And Target Leaders On Rising Tariffs
Apr 23, 2025
Trump Meets With Walmart And Target Leaders On Rising Tariffs
Apr 23, 2025 -
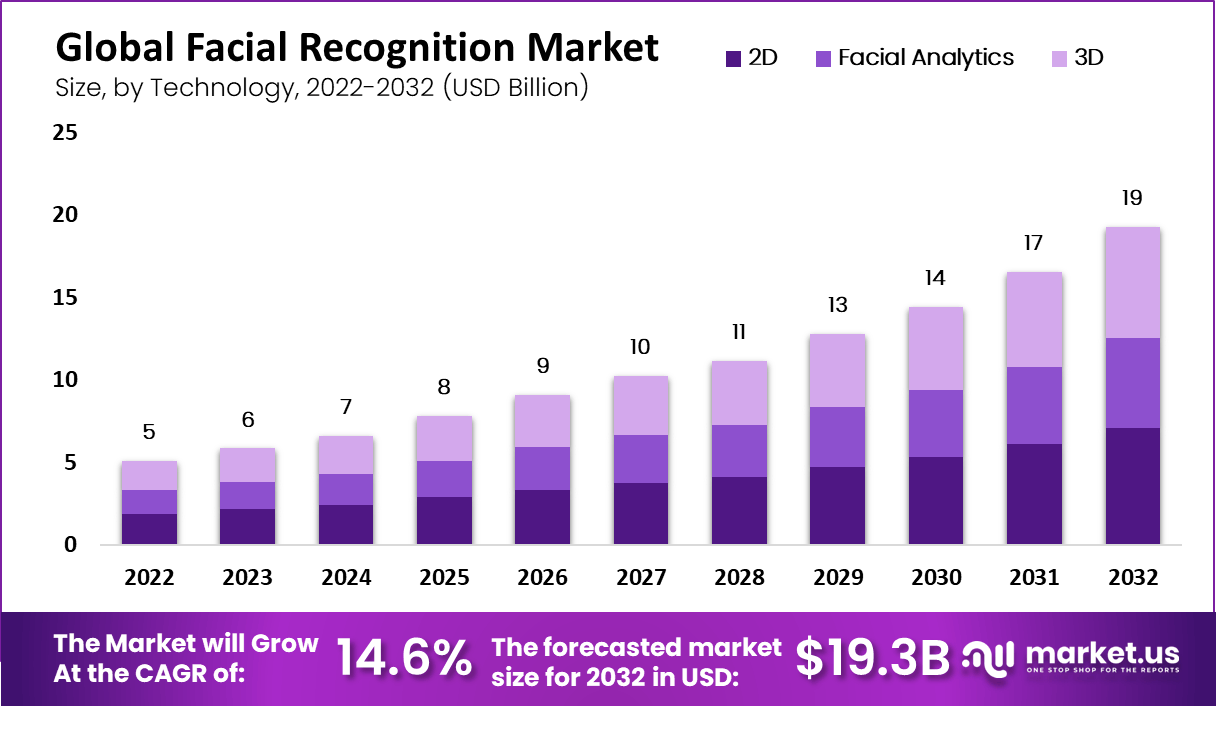 Target Fields Go Ahead Entry Facial Recognition Speeds Up Lines
Apr 23, 2025
Target Fields Go Ahead Entry Facial Recognition Speeds Up Lines
Apr 23, 2025 -
 Calendario Laboral 16 5 Millones De Espanoles Disfrutaran De Un Puente En Abril
Apr 23, 2025
Calendario Laboral 16 5 Millones De Espanoles Disfrutaran De Un Puente En Abril
Apr 23, 2025
